It’s the result of a new non-standard approach
A novel experimental approach has been used to create hundreds of brand-new nanoparticles, intricate materials with never-before-seen properties.
Smaller than 100 nanometers, or approximately the size of a virus, nanoparticles are complex materials with a wide range of potential uses, including medicine, energy, and electronics.
To create materials, chemists typically identify the ideal circumstances for a particular product. By deliberately employing unoptimized conditions to create numerous new materials, a research team at Penn State skipped this strategy though.

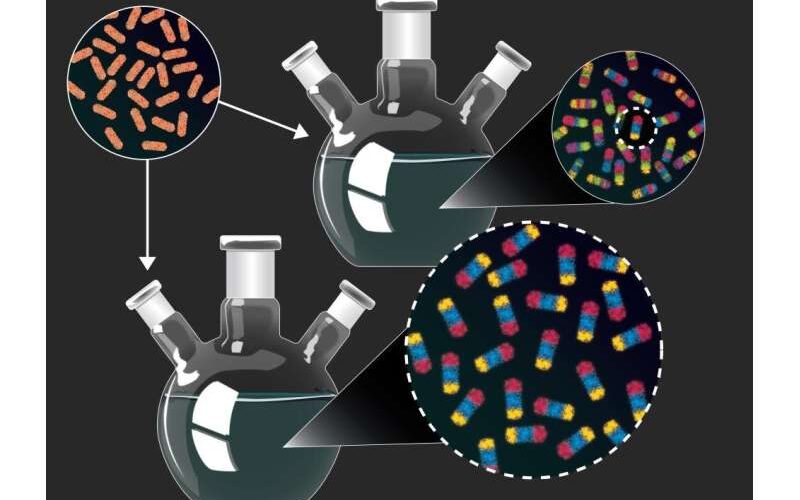
According to this article, researchers begin with straightforward copper sulfide rod-shaped nanoparticles (top left). Then, they use a procedure known as “cation exchange” to swap out some or all of the copper in the particles for other metals. The researchers created and analyzed hundreds of nanoparticles that combine numerous distinct components in diverse combinations in trials that were purposefully not optimized (top), many of which could not have been purposefully manufactured using current design standards. They then rationally produced one of the nanoparticles in high yield using new parameters derived from the initial set of experiments (bottom).
They were able to find unique nanoparticles using this technique, which incorporates a variety of elements in different arrangements. They later conducted further research on these nanoparticles to produce new guidelines that allowed them to create high-yield samples of the most interesting nanoparticle varieties.
It is possible to forecast and construct nanoparticles that could be used to split water using sunlight, identify and treat cancer, and address other significant issues. To work, these particles may need to contain different kinds of semiconductors, catalysts, magnets, and other components, all while adhering to rigid specifications for their size and shape.
“There are a certain number of rules that we and others have developed in this field that allow us to make a lot of different kinds of nanoparticles”, said Raymond Schaak, DuPont Professor of Materials Chemistry at Penn State and the leader of the research team.
“We can also predict, especially with the help of computers, tens of thousands of different nanoparticles that could be really interesting to study, but we have no clue how to make most of them. We need new rules that allow us to make nanoparticles with new properties, new functions, or new applications, and that allows us to better match the speed at which they can be predicted”.
The researchers set up experiments under unoptimized and previously unexplored conditions to see if they could make new types of particles that hadn’t previously been discovered because the current set of rules or design guidelines, available to researchers, limits the variety of nanoparticles that they can produce.
“What we do can be described as ‘discovery without a target'”, said Connor R. McCormick, the paper’s first author and a graduate student in chemistry at Penn State.
“If you have a target in mind, you are trying to modulate the chemistry to make that target, but you need to know what factors to modulate, you need to know the rules, ahead of time. What is so exciting about our approach is that we are letting chemistry guide us and show us what is possible. We can then characterize the products and discover what we can control in order to produce them intentionally”.
The properties of the particles are governed by how the metals are arranged within them and at their interfaces. Typically, this procedure is carried out one metal at a time under experimental circumstances that are specifically tuned to regulate the cation exchange reaction. In this experiment, four separate metal cations were simultaneously supplied under non-optimized circumstances for any given metal cation exchange. After that, they painstakingly used X-ray diffraction and electron microscopy to characterize the resultant particles.
“Unlike most experiments, which are set up to converge on a single product, our goal was to set up the experiment in a way that maximized the diversity of nanoparticles that we produced”, said McCormick. “Of the 201 particles that we analyzed from one experiment, 102 were unique and many of them could not have been produced intentionally using existing design guidelines”.
The researchers next conducted experiments with slightly modified variables, altering the reaction’s temperature or the proportionate quantity and variety of metal cations. As a result, they were able to create even more sophisticated nanoparticles and eventually deduce new rules for the new forms of nanoparticles.
Finally, the team decided on one of the new materials and successfully produced it in greater quantities using the new design principles.
“Eventually, this approach could be used to screen for new particles with specific properties, but currently we are focusing on learning as much as we can about what all is possible to make”, said Schaak. “We’ve demonstrated that this exploratory approach can indeed help us to identify these ‘new rules’ and then use them to rationally produce new complex nanoparticles in high yield”.
Nanoparticles can contribute to a variety of uses, such as drug delivery and targeted therapy; medical imaging and diagnostics; water treatment and purification; energy production and storage (e.g. batteries, solar cells); cosmetics and personal care products; catalysis and chemical reactions; sensors and electronic devices; food packaging and preservation; and environmental remediation.
However, there are also potential risks, such as toxicity and potential harm to human health and the environment; limited biodegradability and potential accumulation in ecosystems, and difficulty in controlling the size and distribution of nanoparticles.